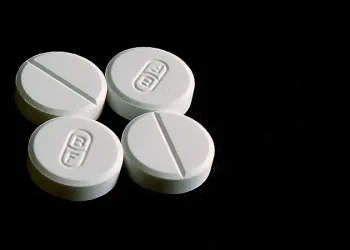
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has issued an urgent warning to parents and caregivers to cease giving Nutrition Ignition Kids Magnesium Glycinate Gummies to children due to the presence of undeclared melatonin, a prescription-only medicine.
This discovery raises significant safety concerns for families across the UK.
Understanding the Risks
Parents in the UK are being urged to stop using Nutrition Ignition Kids Magnesium Glycinate Gummies after tests revealed they contain undeclared melatonin.
Each gummy contains between 1.5 and 1.7 mg of melatonin, exceeding the typical starting dose of 1 mg per day prescribed for children.
This discrepancy poses potential health risks, including drowsiness, headache, dizziness, and nausea.
Safety First
The MHRA’s decision to remove these gummies from sale highlights the importance of regulating supplements marketed as food products but containing medicinal ingredients.
The agency advises parents to dispose of any remaining gummies at pharmacies to prevent accidental ingestion by children.
Potential Effect on Families
This situation disrupts daily routines for families who rely on supplements for their children’s calmness or digestion.
Parents may face additional healthcare visits if side effects occur, while pharmacies become key points for safe disposal.
The incident also prompts online shoppers in the UK to exercise greater caution when purchasing supplements.
Important Considerations
- Melatonin is only authorized in the UK as a prescription medicine for specific sleep disorders in children over six years old.
- The body typically clears melatonin within 12 hours, reducing the likelihood of lasting harm from accidental ingestion.
- The MHRA encourages reporting adverse effects via their Yellow Card scheme to ensure public safety and transparency.
Regulatory Challenges
This incident underscores ongoing challenges in distinguishing between food supplements and medicines within digital marketplaces.
It highlights regulatory gaps that can arise when products are not properly labeled or controlled.
For healthcare professionals, it emphasizes the need for vigilance when advising parents about supplement use.
Additional Reading
To Sum Up
This alert serves as a crucial reminder of the importance of transparency and regulation in health products marketed online.
As families navigate these challenges, awareness and informed decision-making become vital tools in safeguarding children’s health against undeclared substances with potential side effects.
Sources: UK Government, MHRA, and Novomins.
Prepared by Ivan Alexander Golden, Founder of THX News™, an independent news organization delivering timely insights from global official sources. Combines AI-analyzed research with human-edited accuracy and context.