The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has today granted a license for lecanemab, a new drug designed to slow the progression of Alzheimer’s disease. This approval marks a significant milestone in the fight against a condition that affects millions worldwide.
Julian Beach, MHRA Interim Executive Director, stated,
“Licensing medicines which meet acceptable standards of safety, quality and efficacy is a key priority for us.”
He added, “We’re assured that, together with the conditions of the licence approval, the appropriate regulatory standards for this medicine have been met.”
How Lecanemab Works
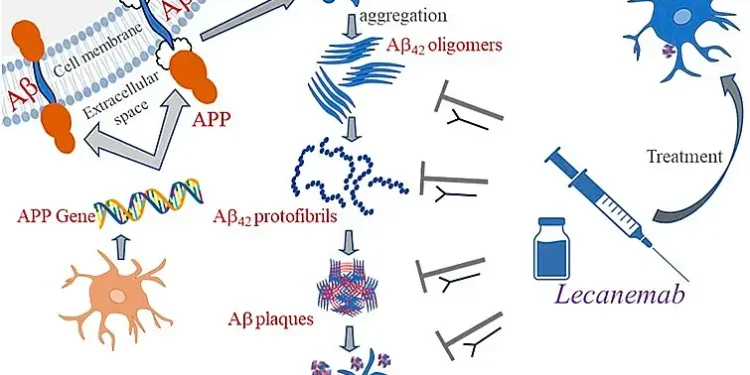
Lecanemab is a monoclonal antibody that targets amyloid beta, a protein that forms plaques in the brains of Alzheimer’s patients. By reducing these plaques, the drug aims to slow the disease’s progression.
Important Facts about Lecanemab:
- Administered intravenously every two weeks
- 10mg/kg dose given over approximately one hour
- Treatment continues until progression to moderate Alzheimer’s
Who Can Receive the Treatment?
The MHRA has approved lecanemab for adults in the early stages of Alzheimer’s disease. However, there’s a crucial caveat: patients must have either one or no copies of the apolipoprotein E4 gene (ApoE4).
This genetic distinction is significant because:
- 15% of Alzheimer’s patients have two copies of ApoE4 (homozygous)
- Homozygous patients are at higher risk of side effects
- Benefit for homozygous patients is uncertain compared to others
Safety Concerns and Monitoring
While the approval offers hope, it comes with important safety considerations. The most common side effects include infusion-related reactions, headaches, and Amyloid Related Imaging Abnormalities (ARIAs).
ARIAs can manifest as:
- Temporary brain swelling (ARIA-E)
- Small spots of bleeding in the brain (ARIA-H)
The MHRA emphasizes ongoing safety monitoring, with Beach noting,
“As with all medical products, we will keep its safety under close review.”
Next Steps and Access
Lecanemab’s introduction will be carefully managed by health authorities.
- A central registration system will control access
- A post-authorisation safety study will be conducted
- Patients on anticoagulants or with cerebral amyloid angiopathy are excluded
For those eager to learn more about lecanemab and its availability, visit the MHRA Products website for detailed information on the Summary of Product Characteristics and Patient Information leaflets.
Sources: THX News & Medicines and Healthcare products Regulatory Agency.